Author: Dr. Maricel Maffini, Consultant / Publication date: June 10, 2024

what happened
The Good Food Foundation recently released its annual awards, which recognize foods that have both great taste and responsible business practices, but a plant-based blue cheese product was controversially named as a finalist in the cheese category, only to be disqualified and removed from the final list. According to the foundation, the product was disqualified because one of its ingredients, kokum butter, is not GRAS (Generally Recognized As Safe) by the Food and Drug Administration.
Why is this important?
Kokum butter is extracted from the seeds of the fruit of the kokum tree, which is grown primarily in India. The substance is not on the FDA’s list of approved or safety-reviewed ingredients. Someone, somewhere, has determined that kokum butter is GRAS for use in food. But who, when, and on what basis made that determination is unclear. For example, how much is safe to eat? Is it safe for anyone, including children, pregnant women, and people with pre-existing medical conditions? Can it cause an allergic reaction or interfere with medications? Does it get quickly eliminated from the body? Does it mimic or interfere with hormones? We don’t know, and neither does the FDA.
Here is a brief overview of the GRAS system, which we have covered in detail in the Broken GRAS blog.
“General Recognition” means that a safety evaluation has been conducted and published, and the scientific community agrees that it is safe for use in food. GRAS substances are not subject to premarket approval by FDA. FDA has interpreted the law to mean that manufacturers can independently determine that a substance’s use is GRAS without notifying the agency. FDA has created a voluntary program for manufacturers to submit their chemical safety determination in the form of a GRAS notice to the agency for review. Manufacturers can withdraw their request for review at any time and still claim that their product is GRAS. See the decision tree below.
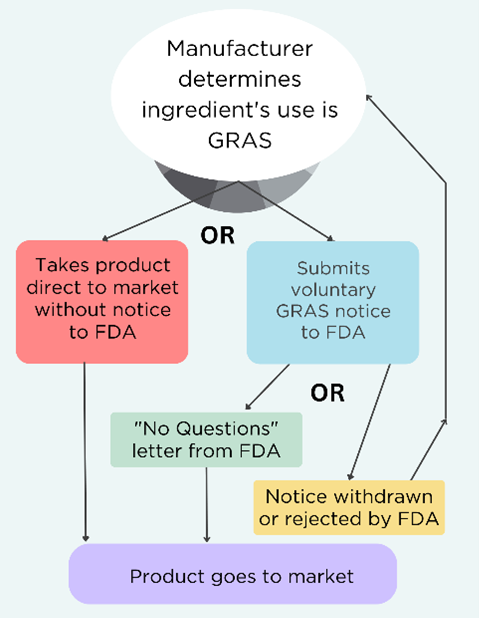
Our take
In 2022, more than 400 people were made sick by cod flour, another ingredient whose safety is unknown. Like kokum butter, cod flour has not been approved or reviewed for safety by the FDA.
We applaud the Good Food Foundation for mandating FDA review of ingredients used in award-winning foods. This is a matter of protecting public health. We fully support, at a minimum, the submission of GRAS notices by companies and their review by FDA. While we have criticized FDA’s outdated science in evaluating the safety of chemicals, these notices provide a level of visibility into the food supply that would not otherwise be available to the agency charged with protecting the public.
Next steps
We will continue to work with the FDA to ensure that the agency has the tools and resources to increase oversight of companies that make GRAS claims without disclosing safety evaluations. The ongoing restructuring of the FDA and the creation of the Human Food Program are a great opportunity to fix the dysfunctional GRAS program and ensure that all Americans can have confidence in the safety of the food they eat.
This entry was posted in Broken GRAS, FDA, Food, Public Interest. Author: drmvma. Bookmark the permalink. Trackbacks are closed, but you can still trackback.
Source link


